Authors: Lauren Radlinski, Sarah E. Rowe, Laurel B. Kartchner, Robert Maile, Bruce A. Cairns, Nicholas P. Vitko, Cindy J. Gode, Anne M. Lachiewicz, Matthew C. Wolfgang, and Brian P. Conlon
Journal: PLoS Biology
PMID: 29176757
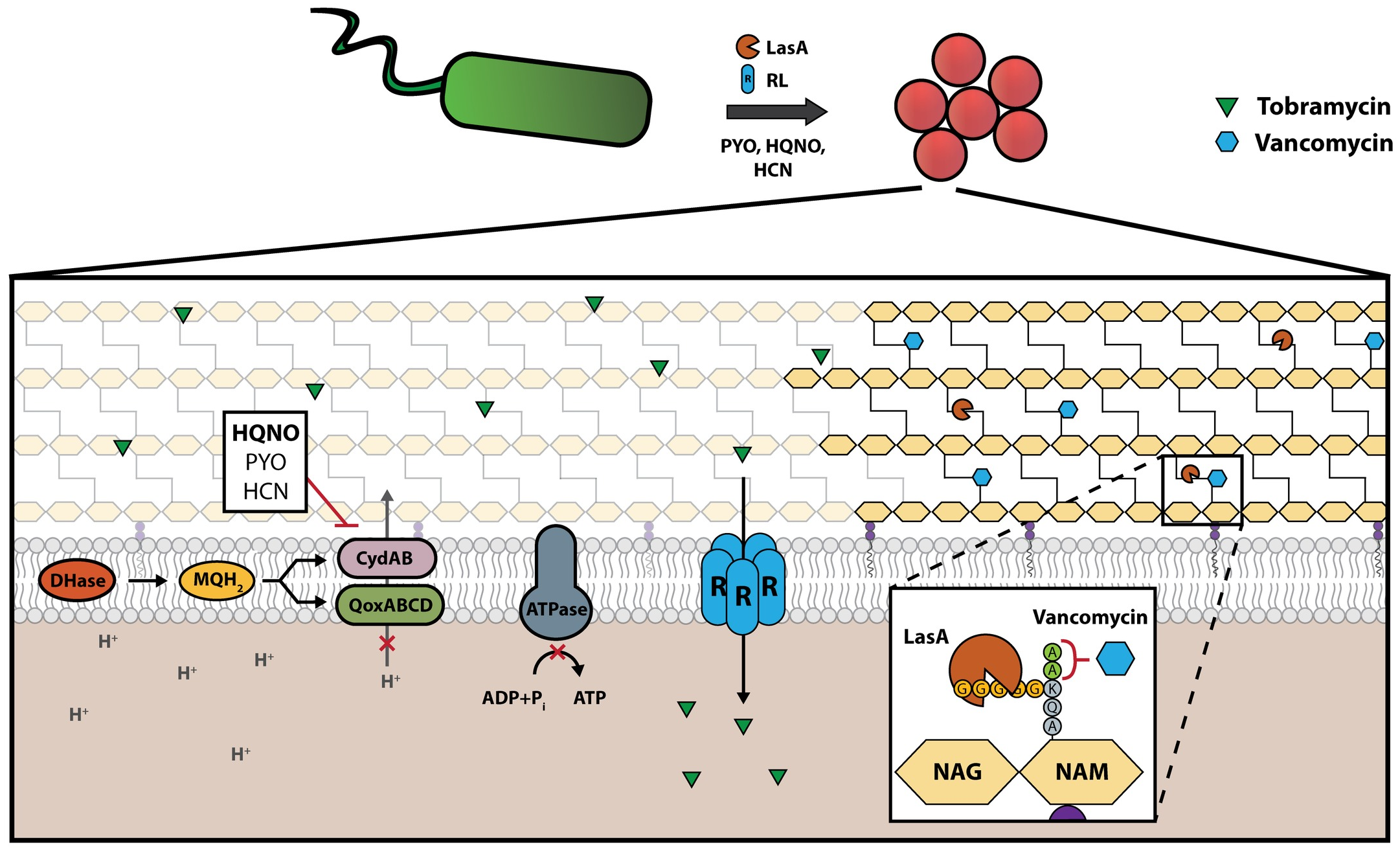
Accurate prediction of antimicrobial efficacy is essential for successful treatment of a bacterial infection. While many studies have considered the impacts of genetically encoded mechanisms of resistance, nongenetic determinants of antibiotic susceptibility during infection remain poorly understood. Here we show that a single interspecies interaction between 2 human pathogens, S. aureus and P. aeruginosa, can completely transform the antibiotic susceptibility profile of S. aureus. Through multiple distinct mechanisms, P. aeruginosa can antagonize or potentiate the efficacy of multiple classes of antibiotics against S. aureus. We identify the exoproducts responsible for altering S. aureus susceptibility to antibiotic killing, and furthermore demonstrate that these compounds are produced at varying levels in P. aeruginosa clinical isolates, with dramatic repercussions for S. aureus antibiotic susceptibility. Finally, we use a mouse model of P. aeruginosa–S. aureus coinfection to demonstrate that the presence of P. aeruginosa significantly alters the outcome of S. aureus antibiotic therapy in a host. These findings indicate that the efficacy of antibiotic treatment in polymicrobial infection is determined at the community level, with interspecies interaction playing an important and previously unappreciated role.